Clinical Trials for Ages 2 to <6 Years
TRIAL 6 (KIWI): Patients with CF age 2 to less than 6 years. KALYDECO® (ivacaftor): Safety consistent with patients age 6 years and older1,2
ON THIS PAGE: Study Design | Summary of Results
Mutations Eligible for Study2 (mutations in bold were enrolled)
G551D, G1244E, G1349D, G178R, G551S, G970R*, S1251N, S1255P, S549N, or S549R
*KALYDECO is not indicated for use in patients with a G970R mutation1
STUDY DESIGN1,2
- Trial 6 was a 24-week, open-label trial (N=34) evaluating safety, pharmacokinetics, and pharmacodynamics in patients with CF age 2 to <6 years (mean age: 3 years)1
- Patients were eligible if they had either a G551D, G1244E, G1349D, G178R, G551S, G970R*, S1251N, S1255P, S549N, or S549R mutation2
- Of the 34 patients enrolled, 32 had the G551D mutation and 2 had the S549N mutation2
- Patients received KALYDECO every 12 hours with fat-containing food, in addition to their prescribed CF therapies, based on weight; 7 kg to <14 kg: One 50 mg packet of oral granules; ≥14 kg: One 75 mg packet of oral granules2
Primary outcome2: Safety, assessed by adverse events and clinical laboratory assessments
Select secondary outcome measure2: Absolute change from baseline in sweat chloride concentration at 24 weeks
Trial 6 limitations2
- The study was open label and not placebo controlled; therefore, causality cannot be attributed
- This study was unblinded, which may have introduced a treatment bias
SUMMARY OF RESULTS1
Safety results and pharmacokinetics were similar to older patients1
- The safety profile for patients in Trial 6, including type and frequency of adverse reactions, was similar to that observed in Trials 1 and 2
- Transaminase elevations were more common in patients who had abnormal transaminases at baseline
- The incidence of patients experiencing transaminase elevations (ALT or AST) >3 x ULN was 14.7% (5/34). All 5 patients had maximum ALT or AST levels >8 x ULN, which returned to baseline levels following interruption of KALYDECO dosing. KALYDECO was permanently discontinued in 1 patient
- Ivacaftor exposure in patients age 2 to <6 years administered either 50 mg or 75 mg of KALYDECO oral granules every 12 hours was similar to that observed in older patients administered KALYDECO tablets
- KALYDECO oral granules (2 x 75 mg) had similar bioavailability as the 150 mg tablet when given with fat-containing food
The efficacy of KALYDECO in patients age 2 to <6 years
was extrapolated from patients age 6 years and older with support from
population pharmacokinetic analyses showing similar drug exposure levels in adults and
children age 2 to <6 years.1
Reduction in sweat chloride from baseline (pharmacodynamic measure) with KALYDECO1,†
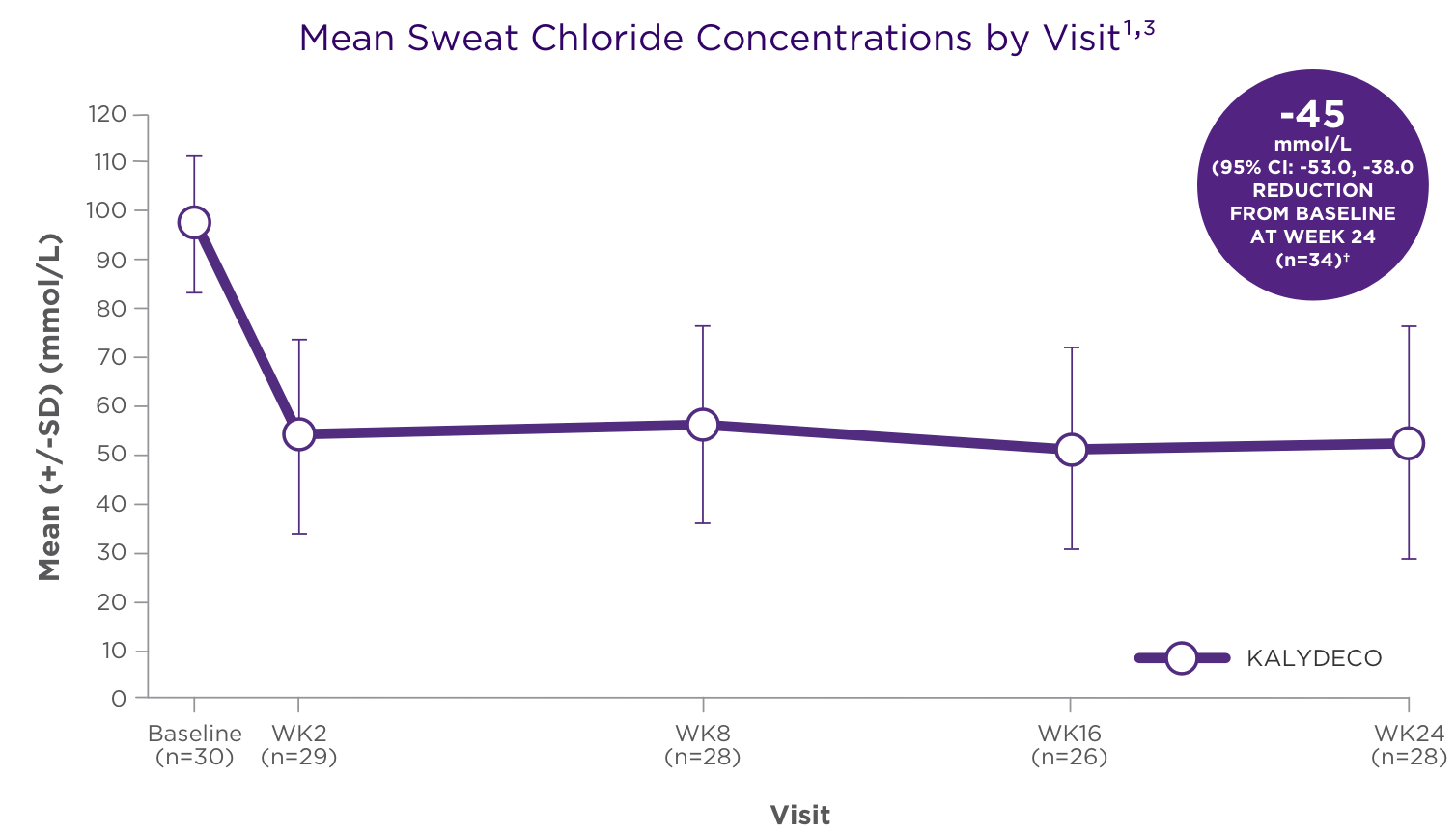
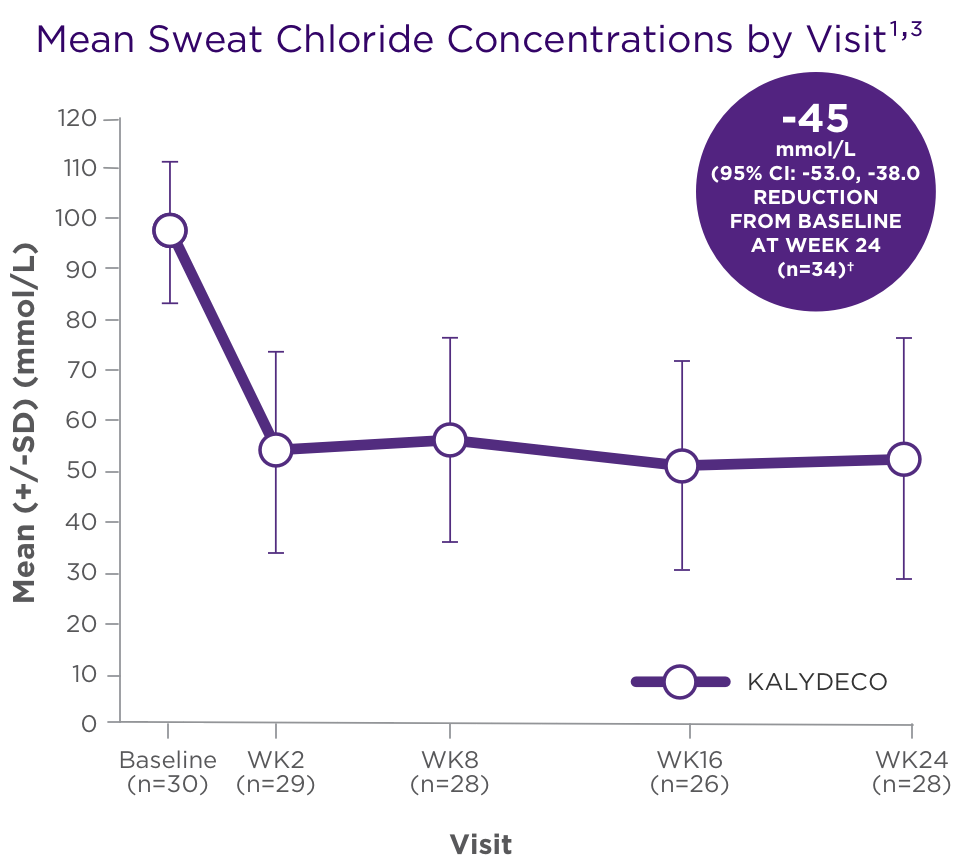
†SD 24.3, P=0.002 for patients who received 50 mg; SD 27.6, P<0.0001 for patients who received 75 mg.2
- The mean absolute change from baseline in sweat chloride for patients age 2 to <6 years (n=34) was −45 mmol/L (95% CI: −53, −38) through Week 24 (secondary endpoint)1,2
- There was no direct correlation between decrease in sweat chloride levels and improvement in lung function (FEV1)1
CI, confidence interval; SD, standard deviation.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Transaminase (ALT or AST) Elevations
- Elevated transaminases have been reported in patients with CF receiving KALYDECO. Transaminase elevations were more common in patients with a history of transaminase elevations or in patients who had abnormal transaminases at baseline. ALT and AST should be assessed prior to initiating KALYDECO, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of transaminase elevations, consider more frequent monitoring of liver function tests
- Patients who develop increased transaminase levels should be closely monitored until the abnormalities resolve. Dosing should be interrupted in patients with ALT or AST of greater than 5 times the upper limit of normal (ULN). Following resolution of transaminase elevations, consider the benefits and risks of resuming KALYDECO
INDICATIONS AND USAGE
KALYDECO is indicated for the treatment of cystic fibrosis (CF) in patients age 1 month and older who have at least one mutation in the CFTR gene that is responsive to ivacaftor potentiation based on clinical and/or in vitro assay data.
If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use.
INDICATIONS AND USAGE
KALYDECO is indicated for the treatment of cystic fibrosis (CF) in patients age 1 month and older who have at least one mutation in the CFTR gene that is responsive to ivacaftor potentiation based on clinical and/or in vitro assay data.
If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of a CFTR mutation followed by verification with bi-directional sequencing when recommended by the mutation test instructions for use.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Transaminase (ALT or AST) Elevations
- Elevated transaminases have been reported in patients with CF receiving KALYDECO. Transaminase elevations were more common in patients with a history of transaminase elevations or in patients who had abnormal transaminases at baseline. ALT and AST should be assessed prior to initiating KALYDECO, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of transaminase elevations, consider more frequent monitoring of liver function tests
- Patients who develop increased transaminase levels should be closely monitored until the abnormalities resolve. Dosing should be interrupted in patients with ALT or AST of greater than 5 times the upper limit of normal (ULN). Following resolution of transaminase elevations, consider the benefits and risks of resuming KALYDECO
Hypersensitivity Reactions, Including Anaphylaxis
- Hypersensitivity reactions, including cases of anaphylaxis, have been reported in the postmarketing setting. If signs or symptoms of serious hypersensitivity reactions develop during treatment, discontinue KALYDECO and institute appropriate therapy. Consider the benefits and risks for the individual patient to determine whether to resume treatment with KALYDECO
Concomitant Use With CYP3A Inducers
- Use of KALYDECO with strong CYP3A inducers, such as rifampin, substantially decreases the exposure of ivacaftor, which may reduce the therapeutic effectiveness of KALYDECO. Co-administration of KALYDECO with strong CYP3A inducers, such as rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John's wort is not recommended
Cataracts
- Cases of non-congenital lens opacities/cataracts have been reported in pediatric patients treated with KALYDECO. Baseline and follow-up ophthalmological examinations are recommended in pediatric patients initiating treatment with KALYDECO
ADVERSE REACTIONS
Serious Adverse Reactions
- Serious adverse reactions, whether considered drug-related or not by the investigators, which occurred more frequently in patients treated with KALYDECO included abdominal pain, increased hepatic enzymes, and hypoglycemia
Most Common Adverse Reactions
- The most common adverse reactions in the 221 patients treated with KALYDECO were headache (17%), upper respiratory tract infection (16%), nasal congestion (16%), nausea (10%), rash (10%), rhinitis (6%), dizziness (5%), arthralgia (5%), and bacteria in sputum (5%)
- The safety profile for the CF patients enrolled in clinical trials (Trials 3-8) was similar to that observed in the 48-week, placebo-controlled trials (Trials 1 and 2)
USE IN SPECIFIC POPULATIONS
Pediatric Use
- The safety and effectiveness of KALYDECO in patients with CF younger than 1 month of age have not been established. The use of KALYDECO in children under the age of 1 month is not recommended
- Use of KALYDECO in patients aged 1 to less than 6 months born at a gestational age less than 37 weeks has not been evaluated
Click here to access full Prescribing Information.
References:
1. KALYDECO [prescribing information]. Boston, MA: Vertex Pharmaceuticals Incorporated; August 2023. 2. Davies JC, Cunningham S, Harris WT, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2-5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4(2):107-115. 3. Data on File. Vertex Pharmaceuticals Incorporated. Boston, MA. REF-0084 (v1.0); 2018.

